Recently, a new version of ISO 17511 has been published, bringing forth updated requirements for ensuring metrological traceability in In-vitro Diagnostic Medical Devices (IVDs). Within the realm of IVD devices, we have previously explored various topics, including ISO 15189, ISO 23640, which pertains to device stability, performance evaluation plans, and EU Reference laboratories under IVDR 2017/746.
Before delving into the specific requirements outlined in the standard, it is crucial to establish a clear understanding of what metrological traceability entails. As defined in the International Vocabulary of Metrology, traceability refers to the property of a measurement result that can be linked to a reference point through a documented unbroken chain of calibrations, with each calibration contributing to the overall measurement uncertainty. In essence, it is the ability to establish a verifiable connection between a measurement and a recognized reference, ensuring accuracy and reliability.
Equally important is grasping the concept of calibration within the context of traceability. Calibration involves utilizing the value of a previous reference material in the traceability hierarchy to assign a value, through measurement, to the next calibrator in the hierarchy. Typically, this involves procuring certified reference materials at different concentrations, which are then employed to calibrate a linear measurement equation utilized for accurate measurements.
Now, let us explore the detailed requirements outlined in the new version of ISO 17511. By examining these requirements, we can gain valuable insights into the necessary measures for achieving metrological traceability in In-vitro Diagnostic Medical Devices.
Traceability Hierarchies According to ISO 17511
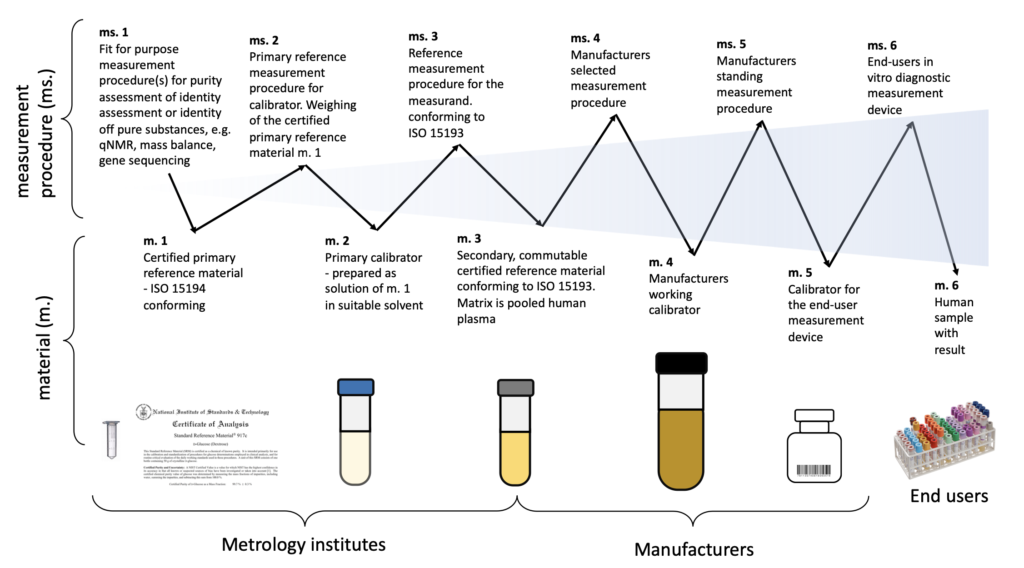
ISO 17511 provides a comprehensive framework that encompasses six distinct calibration hierarchies and value transfer models. The specific hierarchy adopted depends on the availability of traceability to the International System of Units (SI).
Within the traceability hierarchy, calibration plays a crucial role. It involves utilizing the value of the previous reference material to assign a value through measurement to the subsequent calibrator in the hierarchy. This step-by-step process ensures the accuracy and reliability of measurements in the calibration chain.
Let’s explore the six material types that constitute the calibration hierarchies:
- Certified Primary Reference Material: These materials adhere to the specifications outlined in ISO 15194. They serve as the foundation of the calibration hierarchy, providing a trusted reference point for subsequent calibrators.
- Primary Calibrator: This is a solution prepared using a suitable solvent and containing the material of interest at a known concentration (designated as m. 1). It serves as an intermediate calibrator within the hierarchy.
- Secondary, Commutable Certified Reference Material: These materials, conforming to ISO 15193, are characterized by their ability to exhibit comparable properties to the human samples being tested. Pooled human plasma serves as the matrix for these reference materials.
- Manufacturers’ Working Calibration: This calibration level involves the specific calibration performed by the manufacturers of the measurement devices. It ensures the accuracy and reliability of the devices before they are made available to end-users.
- Calibrator for the End-User Measurement Device: This calibration level focuses on the calibration of the measurement device used by end-users. It guarantees the accuracy and reliability of measurements within the intended operational environment.
- Human Sample with Result: This final calibration level involves the utilization of actual human samples with known results. These samples provide a real-world benchmark for evaluating the performance and accuracy of the measurement system.
By incorporating these distinct material types and calibration hierarchies, ISO 17511 establishes a robust framework for achieving and maintaining traceability in In-vitro Diagnostic Medical Devices.
he calibration hierarchy extends further with the inclusion of user calibrators, which contribute to the overall accuracy and reliability of measurements within the system.
To ensure clear understanding and documentation of the calibration hierarchy, the technical documentation should incorporate a visual representation, such as a figure or illustration, that depicts the linkage from the final results obtained using human samples and the specified measuring system, up to the highest metrological reference available.
Each step within the calibration hierarchy necessitates the identification of the measured quantity in the relevant reference material or human samples (in the case of final measurements). Additionally, establishing the relationship between the measured quantity (or quantities) and the measurand is essential.
Certain prerequisites must be met to effectively implement the calibration hierarchy:
- Traceability to SI: The calibration hierarchy must be traceable to the International System of Units (SI) to ensure consistency and accuracy in measurements.
- Availability of Certified Reference Materials: Certified reference materials that meet specific criteria and standards should be readily accessible within the calibration hierarchy.
- Availability of Reference Measurement Procedures: Proper reference measurement procedures must be in place to facilitate accurate value assignment at each step of the calibration hierarchy.
- Availability of Harmonization Protocols: Harmonization protocols play a vital role in ensuring uniformity and consistency across different measurement systems and laboratories.
Primary reference materials, typically characterized by high levels of purity, play a crucial role within the calibration hierarchy. They contain analytes that are well-defined in terms of their physiochemical properties and undergo thorough evaluation for compositional stability and integrity. Accompanied by a certificate, these materials are referred to as certified reference materials (CRMs).
A primary calibrator is then prepared using a primary reference material and assigned a value through a primary reference measurement procedure. This step establishes a reliable baseline for subsequent calibration activities.
For the transfer of value from a primary calibrator to a secondary calibrator or secondary reference material, an appropriate reference measurement procedure specific to the measurand must be employed. This ensures the accuracy and consistency of measurements throughout the calibration hierarchy.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
