The Declaration of Conformity is one of the key document asked to be prepared by medical device manufacturer in order to declare the compliance of their products and related quality system to the requirements of the European Medical Device Regulation.
We have already been discussing about many aspects of the EU Medical Device Regulation, mainly related to post-market surveillance and technical documentation, but also related to many other aspects such as General Safety and Performance Requirements, requirements for the Person Responsible for Regulatory Compliance and the Strategy for Regulatory Compliance.
General Requirements
The general requirements for the Declaration fo conformity is described in the Article 19 of the regulation EU MDR 2017/745. As we have been mentioned before, the declaration of conformity shall clearly state that the requirements mentioned in the regulation has been fully met for the medical devices brought to the European market.
Moreover, the first paragraph of Article 19 clearly mentioned that the declaration for conformity shall be continuously updated and shall be translated into an official Union language or languages required by the Member State(s) in which the device is made available. This basically means that, potentially, if the device is made available in every EU Country, then the declaration shall be translated in all the official languages of the European Union.
Contents of the EU Declaration of Conformity
The mandatory contents of the EU declaration of Conformity are defined in the Annex IV of the EU MDR 2017/745.
The summary of the contents of the Declaration of Conformity is reported in the scheme below:
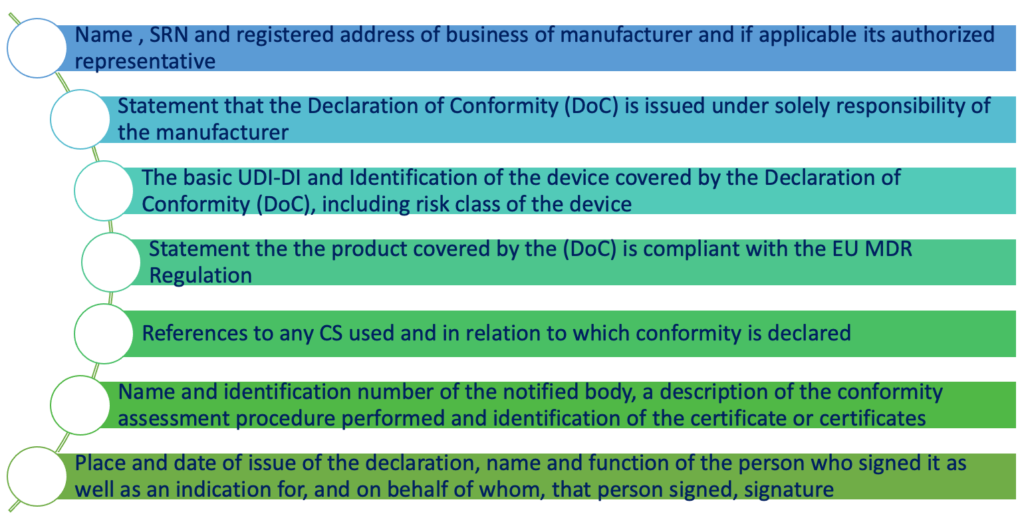
Regarding the first point, it is important to mention the single registration number (SRN) which is regulated by article 31 of the EU Medical Device Regulation. In fact, always according to Article 31, the manufacturer shall use the SRN when applying to a notified body for conformity assessment. In order to obtain the SRN, the manufacturer shall submit information mentioned in the Annex IV to the competent authority which will issue the SRN after having verified the correctiveness of the submitted information.
Place and date of issue of the declaration, name and function of the person who signed it as well as an indication for, and on behalf of whom, that person signed, signature.
Multiple Declarations of Conformity
There is the possibility that devices shall be compliant with specific requirements of other European regulation that also requires the presence of a Declaration of Conformity. In this specific case, Article 19 defines that it is possible to have a single EU Declaration of Conformity in relation of all EU regulation applicable to the specific device. The declaration shall contain all the information required for identification of the Union legislation to which the declaration relates.
Declaration of Conformity and Manufacturer Responsibility
While drawing up the EU Declaration of conformity, the manufacture assumes the responsibility for the compliance of the requirements of the EU Medical Device Regulation 2017/745. For this reason it is very important the declaration is signed by top management of the organization with legal responsibility.
However, it is important to mention that according to Article 15, it is responsibility of the Person Responsible for regulatory Compliance to technical documentation and EU Doc are kept up to date and the information mentioned therein is correct.
Other Requirements
There are as well other requirements related to the DoC. For example, article 10 defines the retention time for the declaration as follows:
Manufacturers shall keep the technical documentation, the EU declaration of conformity and, if applicable, a copy of any relevant certificate, including any amendments and supplements, issued in accordance with Article 56, available for the competent authorities for a period of at least 10 years after the last device covered by the EU declaration of conformity has been placed on the market. In the case of implantable devices, the period shall be at least 15 years after the last device has been placed on the market.
Basically the retention time of the EU DoC is the same of the technical documentation and the related CE certificate, thus 10 years after the last device e has been placed on the market; 15 years in case of implantable devices.
Declaration of Conformity Template
4EasyReg has prepared a template for EU Declaration of Conformity fully compliant with Article 19 and Annex IV of the European Medical Device Regulation 2017/745. This is a word document, thus completely editable, it is ready to be modified to properly include the data of your organization.
Do not hesitate to download this document to avoid any issue in relation to the compliant of the DoC with the applicable requirements of the regulation.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!

