The Integral Role of Lifetime in Medical Device Utilization
The lifetime of a medical device is a critical period during which the tool must safely and reliably perform its intended function. This encompassing term, medical device lifetime, signifies more than the duration a device is operational; it embodies the intersection of safety, performance, and regulatory adherence. This concept is of paramount importance as it drives decision-making among manufacturers, operators, and consumers alike, and directly impacts patient safety. Consequently, understanding, establishing, and differentiating the medical device lifetime along with its consequences if ignored is essential for all actors in the healthcare sector.Recently, Team NB published a position paper on Medical Device lifetime to further clarifies this important aspects of medical devices. This article moves in the direction to provide an in depth overview of the regulation of medical devices in Europe and United States, such as post-market surveillance, risk management, Regulatory compliance and technical documentation.
Understanding Medical Device Lifetime Significance
Defining the medical device lifetime is pivotal for safety and efficacy. Manufacturers must integrate the anticipated lifetime into the device’s intended purpose. This foresight is essential within risk management processes, such as those detailed in Annex I of the Medical Device Regulation (MDR) and the ISO 14971 standard, to ensure devices maintain their safety and intended performance throughout their service life.
Devices operated beyond their established service life pose severe risks. Age-related decay, like the brittleness of plastics or malfunction of electronic components due to repeated use, can lead to a compromise in a device’s safety and performance—threatening patient health and bringing potential legal challenges against all parties involved.
For manufacturers, predicting the medical device lifetime influences component, material, and technology choices during development. Operators lean on these predictions to determine end-of-life actions for devices, such as decommissioning units that appear operational but may no longer meet safety standards. From a consumer’s perspective, medical device lifetime may factor heavily into purchasing decisions, directly influencing the competitive market.
Establishing Medical Device Lifetime Parameters
The lifetime of a device is dictated by its ability to operate safely within its design specifications. The determination of this period is multifaceted, assessing factors such as wear and tear, technological advancements, and historical performance data amidst normal operational stresses. Regulatory guidelines impose the responsibility of defining this period explicitly on manufacturers.
The MDR does not pinpoint what constitutes device lifetime; however, it emphasizes the importance of a device not compromising health or safety “during the lifetime of the device, as indicated by the manufacturer,” under normal usage and proper maintenance. This highlights the manufacturer’s obligation to specify the lifetime, guiding the selection of device components and informing the necessary service intervals.
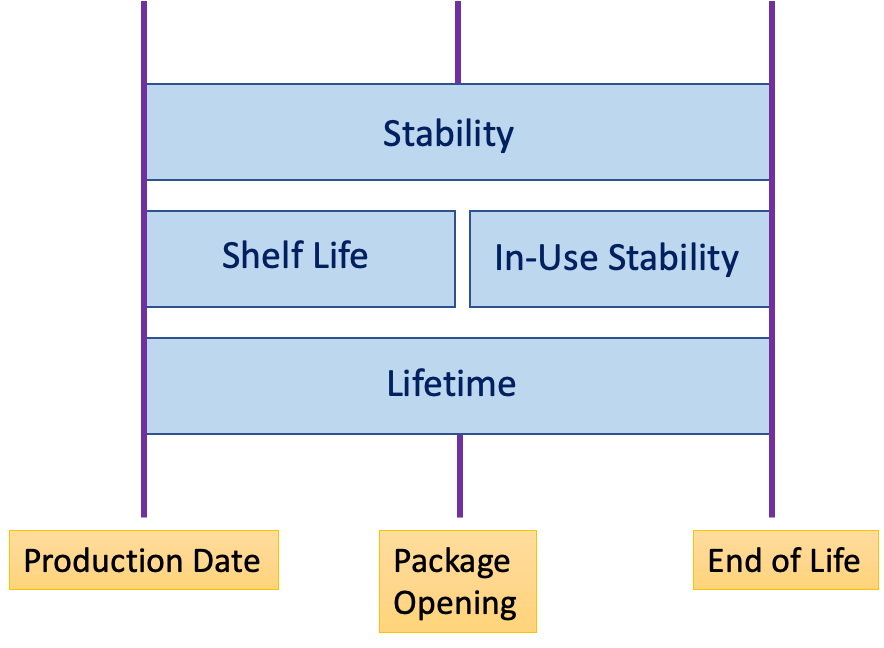
A comprehensive understanding of a medical device’s design— including component lifespan and material durability—is necessary to indicate its lifetime. The sophistication of technology used and the robustness of materials all contribute to defining the maximum service life of a medical product.
Distinguishing Lifetime from Related Concepts
Misunderstandings often arise when conflating medical device lifetime with terms such as shelf life or operating time. These concepts, while related, represent different aspects of a device’s usability. Shelf life refers to the period a device is usable from manufacture to initial operation, whereas operating time incorporates the actual duration of active use.
Another distinction must be made between the end of service and the expected lifetime. End of service life indicates when a device is no longer supported or is required to be decommissioned, while lifetime expectancy considers the anticipated operational lifespan, which can be influenced by usage frequency and maintenance practices.
Frequency of use is intrinsically linked to the degradation and potential life expectancy of a device. Manufacturers must account for the varying degrees of operation, from sporadic to near-constant, in their lifetime specifications, justifying the creation of robust designs capable of enduring high usage demands.
Compliance and Implications of Medical Device Lifetime
Manufacturers bear the crucial role of specifying the lifetime of a medical device, which entails an extensive analysis of the risks across its entire period of use. Through stringent risk assessments, appropriate documentation, and transparent communication, manufacturers ensure a device’s longevity is clear and understandable.
Healthcare providers must navigate the operational challenges posed by the end of a medical device’s service life. Despite indications that a device might function without issue past its designated lifetime, the manufacturer’s stipulated parameters take precedence, often mandating the retirement of the device.
Medical device lifetime is not only a technical detail but also a selling point. For budget-conscious customers, the longevity of a device can tip the scales during procurement, influencing health facilities to opt for devices promising extended service life over comparable alternatives.
Conclusions
In conclusion, the notion of lifetime in the context of medical devices is complex. However, the understanding and adherence to the established lifetime are vital to ensure that patients and users remain safe and that the medical device performs as intended over the course of its life. It is a shared responsibility among manufacturers, operators, and customers, underscoring the collective goal to uphold the highest standards of healthcare delivery.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
