Implantable medical devices are usually highly risky devices for which a potential failure may cause serious consequences to the patient. This is the reason why a great attention has been brought by the regulator to the safety of these types of devices, especially in the new European Medical Device Regulation 2017/745.
In this article, we will go through the main requirements associated to implantable medical devices according to the European regulation.
Giver the particularity of these types of devices, there are specific requirements that need to be taken in consideration and for which evidences of compliance shall be provided within the medical device file or with other type of documentation, for example in the contest of the Strategy for Regulatory Compliance.
What is an Implantable Medical device?
The definition of implantable medical device that is provided in the EU MDR text is the following:
‘implantable device’ means any device, including those that are partially or wholly absorbed, which is intended:
- to be totally introduced into the human body, or
- to replace an epithelial surface or the surface of the eye
by clinical intervention and which is intended to remain in place after the procedure.
Any device intended to be partially introduced into the human body by clinical intervention and intended to remain in place after the procedure for at least 30 days shall also be deemed to be an implantable device.
General Considerations for Implantable Devices
As any other medical devices, implantable medical devices can be classified as active or passive devices. A passive device does not have a source of energy in the device, and the device can be activated through other means like movements or breathing of the patient.
Instead, active implantable medical devices are powered devices that are inserted into a patient’s body, through either a natural orifice or by surgical means, and are intended to remain in the patient’s body after the procedure.
Active implantable medical devices are considered risky devices for which a high standard of manufacture and a very high level of regulatory compliance need to be achieved.
ISO 13485 Requirements
The ISO 13485:2016 contains several requirements that are associated implantable medical devices that can be summarised on the scheme below. We will go through the main requirements and then we will move towards the requirements mentioned in the European MDR 2017/745.
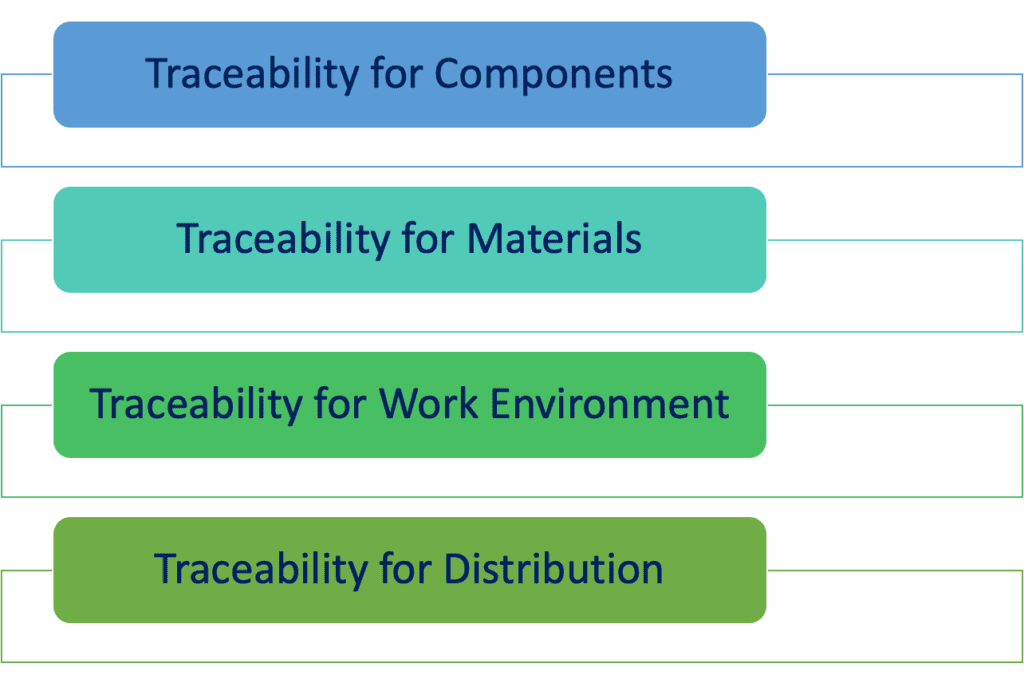
Section 7.5.9.2 provides specific requirements in terms of traceability. Specifically, records for traceability shall be retained for the following topics:
- Components. It is essential that if the devices is made by different components, traceability towards all these component shall ba maintained. Basically each component shall be linked to a well specific BOM and a well specific production record. Doing so, at any moment, it is possible to trace back the component that has been included in a defined device.
- Materials. Any material by which an implantable device is made shall be properly identified and traced; specific evidences that this material is suitable to be used for this type of medical devices shall be provided, as it is a critical aspect for the overall safety of the patient.
- Work environment. As it can be understood, the impact of work environment on implantable device is huge and it is obvious that the specific conditions of work may have a direct impact on the quality and safety of the device. Thus, it is necessary to keep accurate records of the work environment conditions, such as temperature, humidity, etc.
Moreover, an additional requirements for traceability of implantable devices is the following:
The organization shall require that suppliers of distribution services or distributors maintain records of the distribution of medical devices to allow traceability and that these records are available for inspection.
This is related to supplier of distributions services or distributors that are obliged to keep records of the distribution of the devices. This is critical, as there is always the necessity to know the final patient in order to be ready if any safety issue is detected, patient may be quickly contacted.
Finally, one more requirements for implantable medical device is mentioned in the 8.2.6 of ISO 13485:2016, where it is clearly mentioned the necessity to document the identity of personnel performing any inspection or testing on the device during the product release process.
EU MDR 107/745 Requirements
The most important new requirements related to implantable medical device according to EU MDR 2017/745 is the so-called implant card. This requirement goes in the direction to improve the information that is shared with the patient about the implanted device.
The implant card has different purposes, such as:
- Enable the patient to identify the implanted device and to get access to safety-related information.
- Enable patients to identify themselves as persons requiring special care in certain situation, such as security checks or other type of situations.
The requirements associated to the implant card are defined in the Article 18 of the regulation, where the information that need to be present in the implant card are clearly defined. Specifically, the following information shall be included:
- Device name
- Device type
- Unique device identification (UDI) – both the DI and PI shall be included
- Serial number or, where applicable, lot or batch number
- Name and address of the manufacturer of the medical device
- Website of the manufacturer of the medical device
- any warnings, precautions or measures to be taken by the patient or a healthcare professional with regard to reciprocal interference with reasonably foreseeable external influences, medical examinations or environmental conditions, as mentioned in the Article 18 (b)
- Expected life time of the device
- any other information to ensure safe use of the device by the patient.
Moreover, information on the patient needs to be included. This includes names of the patient, implant date and healthcare institution where the device was implanted. This information shall be included at the moment when the card is given to the patient, so to respect as much as possible privacy and GDPR requirements.
Moreover, there are other specific requirements for the implant cards that can be summarised in the scheme below:
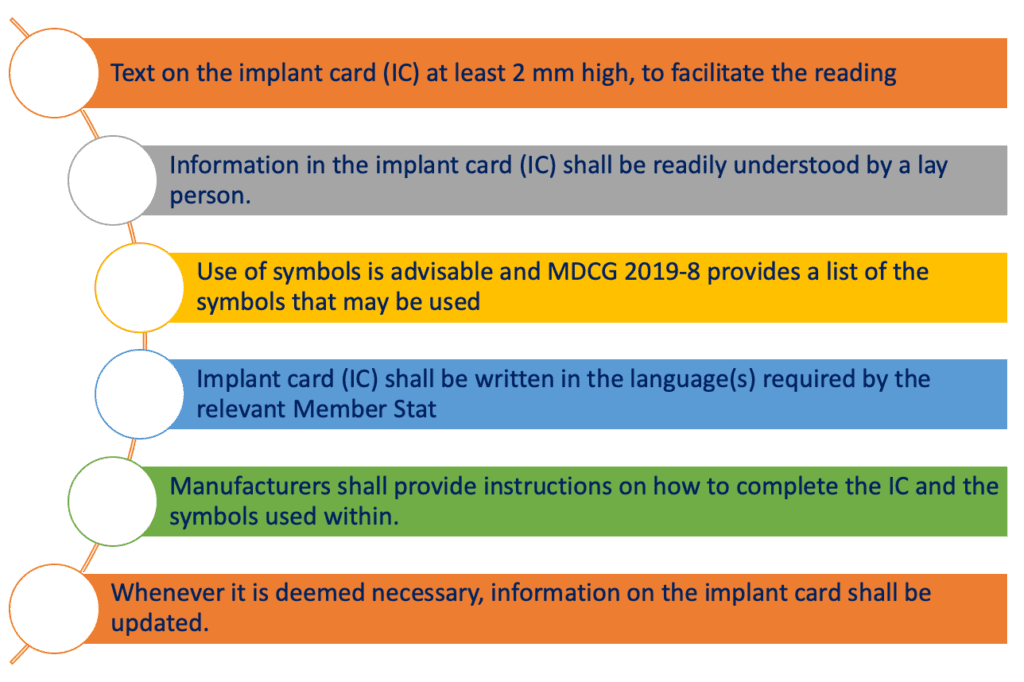
We will not go in details through all these specific requirements, however it is important to mention that MDCG (Medical Device Coordination Group) published a specific guideline MDCG 2019-08 where all the requirements related to the implant card are discussed.
ISO Standards related to Implantable Devices
There are as well other specific standards related to implantable medical devices that need to be properly taken in consideration during the design of the product as well as during production and post-production phase. Here below we can find a list of the most important ISO standards specifically related to implantable devices; it will not be an exhaustive and other ISO standards may be applicable, depending on the type of device.
- ISO 14708-1:2014 – General requirements for safety, marking, and for information to be provided by the manufacturer
- ISO 14708-2:2012 – Cardiac pacemakers
- ISO 14708-3:2017 – Implantable neurostimulators
- ISO 14708-4:2008 – Implantable infusion pumps
- ISO 14708-5:2010 – Circulatory support devices
- ISO 14708-6:2010 – Particular requirements for active implantable medical devices intended to treat tachyarrhythmia (including implantable defibrillators)
- ISO 14708-7:2013 – Particular requirements for cochlear implant systems
- ISO 14117:2012 – Electromagnetic compatibility test protocols for implantable cardiac pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization devices
- ISO 14602:2010 – Non-active surgical implants — Implants for osteosynthesis — Particular requirements
- ISO 14630:2012 – Non-active surgical implants — General requirements
- EC 62304:2006 – Medical device software
Compliance Checklist for Technical Documentation
In order to facilitate the control of the compliance of the technical documentation before submission of the dossier to the notified body, QualityMedDev has prepared a compliance checklist with a detailed list of all the requirements for technical documentation according to MDR 2017/745.
This Technical Documentation Checklist will be an essential tool to simplify the assessment of the conformity of your technical dossier. This 15-pages checklist is provided as a word file, thus fully editable to make it suitable according to the type of product your organization is bringing to the market.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!

