In this comprehensive article, we will delve into the requirements for Trend Reporting as outlined in Article 88 of the EU Medical Device Regulation (MDR) and Article 83 of the EU In Vitro Diagnostic Medical Device Regulation (IVDR). Trend reporting serves as an integral part of post-market surveillance, a topic we have extensively covered in previous posts where we explored periodic safety update reports, post-market surveillance plans, vigilance reporting, and more.
-
 PSUR report Template€64,00
PSUR report Template€64,00 -
 Postmarket Surveillance Plan Template€64,00
Postmarket Surveillance Plan Template€64,00
The primary objective of trend reporting is to systematically monitor and analyze the occurrence of incidents that are not classified as serious incidents over a period of time. By doing so, it aims to assess whether there have been any noteworthy changes in the benefit-risk analysis of the medical device.
The concept of trend reporting is closely intertwined with post-market risk management practices. Its ultimate aim is to effectively manage and mitigate non-serious incidents while ensuring that risk management documentation remains up to date. This proactive approach allows manufacturers and regulatory authorities to maintain a favorable benefit-risk ratio for the device throughout its lifecycle.
By diligently monitoring trends in incident reports, manufacturers can identify patterns or emerging issues that may require further investigation or corrective actions. It enables them to gain valuable insights into the performance, safety, and effectiveness of their devices in real-world settings.
To comply with the regulatory requirements, manufacturers are obliged to establish robust systems for collecting, analyzing, and reporting trend data. This includes defining appropriate metrics, setting thresholds for triggering trend analysis, and establishing clear procedures for evaluating and documenting any changes in the benefit-risk profile.
Furthermore, manufacturers should ensure effective communication and collaboration with competent authorities and other stakeholders to promptly address any emerging trends that may impact the device’s benefit-risk balance. This collaborative approach facilitates the exchange of information and fosters a proactive and transparent approach to patient safety.
For an in-depth overview of the requirements associated to postmarket surveillance according to EU MDR, an online and self-paced training is available at QualityMedDev Academy. This training covers different topics, such as vigilance reporting, postmarket risk management, trend reports and FSCA, and much more.
Guidelines for Trend Reporting Process
The concept of Trend Reporting is not a novel concept and has been incorporated into various guidelines and regulations in the field of medical devices.
One of the key resources providing detailed information on trend reporting is the MEDDEV 2.12, which specifically addresses post-market surveillance requirements for medical devices in the European Union (EU). Although this MEDDEV guideline was developed for the previous medical device directive, it still holds relevance in the absence of a more recent guideline specifically tailored to the post-market surveillance requirements of the Medical Device Regulation (MDR).
Additionally, the Global Harmonization Task Force (GHTF) has developed two guidelines that offer valuable insights into trend reporting. The first guideline focuses on adverse event reporting, while the second one delves into the specific area of trend reporting for adverse events. Both these guidelines contain dedicated sections that provide practical guidance on implementing the trend reporting process effectively.
In a broader sense, trend reports encompass three distinct categories of events:
- Non-serious incidents/events: This category covers incidents that are not classified as serious and are thus included in the trend reporting analysis.
- Adverse events currently exempted from reporting: Certain adverse events may be exempted from immediate reporting requirements but are still considered relevant for trend reporting purposes.
- Adverse events scheduled for periodic reporting: These are adverse events that are captured and reported periodically as part of a predetermined reporting schedule.
However, for the purpose of our discussion, we will primarily focus on trend reports related to non-serious incidents, as this is the central focus of the EU MDR and IVDR.
By closely analyzing trend reports related to non-serious incidents, manufacturers and regulatory authorities gain valuable insights into the overall performance and safety profile of medical devices. These reports enable the identification of patterns or emerging issues that might necessitate further investigation or corrective actions.
Moreover, trend reporting plays a crucial role in ensuring continuous improvement and enhancement of patient safety. By monitoring trends, manufacturers can proactively identify potential risks and take appropriate measures to mitigate them. This ongoing assessment of non-serious incidents helps maintain a positive benefit-risk balance and facilitates the timely implementation of any necessary adjustments to risk management strategies.
Trend Reporting and EU MDR / IVDR
As mentioned earlier, the requirements pertaining to trend reporting are outlined in Article 88 of the EU MDR.
Essentially, this article mandates the activation of trend reporting when there is a statistically significant increase in the frequency or severity of incidents that are not classified as serious incidents, or when expected undesirable side effects arise that could have a substantial impact on the benefit-risk analysis.
To effectively implement an efficient trend reporting process that aligns with the EU regulations, it is imperative to adopt a robust statistical approach that aids in identifying trends warranting reporting.
Naturally, numerous processes can be implemented to construct an effective trend reporting system. In the following sections, we will propose an illustrative approach that can be utilized to identify trends that necessitate reporting.
By utilizing statistical techniques and analysis, manufacturers can identify patterns and deviations in incident data over time. This approach involves applying statistical tools such as trend analysis, time series analysis, or other relevant methods to the collected incident data. By employing these techniques, manufacturers can identify significant changes in incident frequencies or severities that may indicate the need for trend reporting.
Additionally, it is crucial to establish appropriate thresholds for triggering trend reporting. These thresholds should be statistically defined, taking into account factors such as the baseline incident rate, acceptable confidence levels, and significance levels. Manufacturers should determine the thresholds based on sound statistical principles and ensure they are sensitive enough to capture meaningful changes in incident patterns.
Furthermore, it is essential to establish a systematic process for collecting, analyzing, and documenting incident data. This process should encompass robust data management practices, ensuring the accuracy, integrity, and consistency of the collected data. Manufacturers should also maintain clear documentation of the statistical methods used, including assumptions, calculations, and interpretations.
To enhance the effectiveness of trend reporting, manufacturers can consider implementing automated systems or software tools that facilitate data analysis and trend detection. These systems can streamline the process, improve data accuracy, and enable timely identification of reportable trends.
Lastly, it is important to emphasize that the proposed approach serves as an example and can be tailored to suit the specific needs and characteristics of different medical devices and their associated risks. Manufacturers should adapt the approach to their specific context, considering factors such as device complexity, intended use, and the nature of potential incidents.
Practical Approaches for Trend Reporting
One of the viable approaches for identifying trends in non-serious incidents is the utilization of the Product Problem Rate.
The Product Problem Rate can be calculated by considering the number of medical devices (d) present in the market during a specific timeframe (t) and the corresponding number of non-serious events that occur within that same timeframe (t). This rate is determined by taking the ratio of the number of events to the number of devices on the market, considering the designated timeframe.
By calculating and monitoring the Product Problem Rate over time, manufacturers can gain valuable insights into the prevalence and trends of non-serious incidents associated with their medical devices. This approach allows for a quantitative analysis that helps identify variations and fluctuations in the occurrence of such incidents.
When employing the Product Problem Rate, it is crucial to define an appropriate timeframe that aligns with the nature of the device, its expected lifespan, and the frequency of incidents. The timeframe can vary depending on the characteristics of the device and the associated risks. For example, devices with longer expected lifespans or devices used in critical applications may require longer monitoring periods to capture meaningful trends accurately.
To effectively utilize the Product Problem Rate, manufacturers should ensure accurate and comprehensive data collection of both the number of devices on the market and the corresponding non-serious events. This involves implementing robust incident reporting mechanisms, monitoring systems, and quality management processes to capture relevant data accurately.
It is important to note that the Product Problem Rate alone may not provide a complete understanding of the underlying factors contributing to the incidents. Therefore, manufacturers should complement this quantitative analysis with qualitative assessments, such as root cause investigations, feedback from users, and other relevant sources, to gain a comprehensive perspective on the trends observed.
Moreover, manufacturers can enhance the effectiveness of the Product Problem Rate approach by incorporating additional metrics or indicators that capture specific aspects of incident trends. These may include factors such as the severity of incidents, geographical distribution, or correlation with specific device components or usage scenarios. By analyzing these supplementary indicators alongside the Product Problem Rate, manufacturers can derive deeper insights into the nature and potential causes of non-serious incidents.
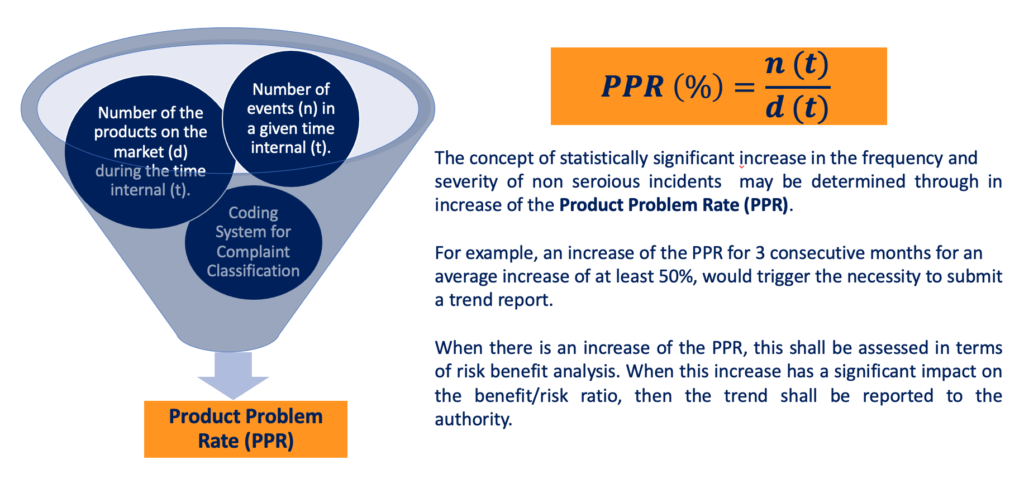
The concept of a statistically significant increase in the frequency and severity of non-serious incidents can be effectively assessed through the measurement of the Product Problem Rate (PPR).
By monitoring the PPR over time, manufacturers can identify noteworthy changes that may indicate an adverse trend. For instance, if the PPR shows a sustained increase over three consecutive months, with an average rise of at least 50%, it could serve as an indication of a concerning pattern that would require reporting.
When an increase in the PPR is observed, it is crucial to assess its implications within the context of risk-benefit analysis. This assessment takes place as part of the comprehensive post-market risk management process, which will be elaborated upon in the final section of this course.
In the context of risk-benefit analysis, the objective is to evaluate the impact of the observed trend on the overall benefit-risk balance of the device. This assessment considers factors such as the severity and potential consequences of the incidents, the intended use of the device, and the benefits it provides to patients and users.
It is important to note that the evaluation of an increase in the PPR should not be viewed in isolation. It should be complemented by other relevant information and data, including feedback from healthcare professionals and users, clinical studies, and relevant scientific literature. These additional insights contribute to a more comprehensive understanding of the observed trend and enable informed decision-making regarding the reporting and management of non-serious incidents.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!


